The periodic table is way of ordering the chemical elements by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
Chemist Dimitri Mendeleev started the development of the table in 1869. He predicted the discovery of other elements, and left spaces open for them.
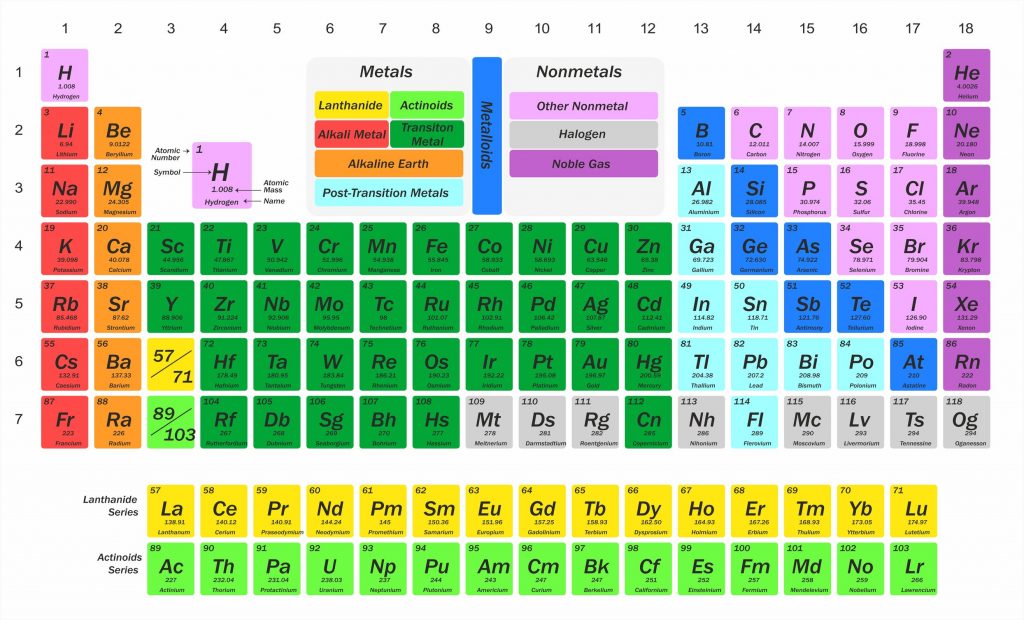
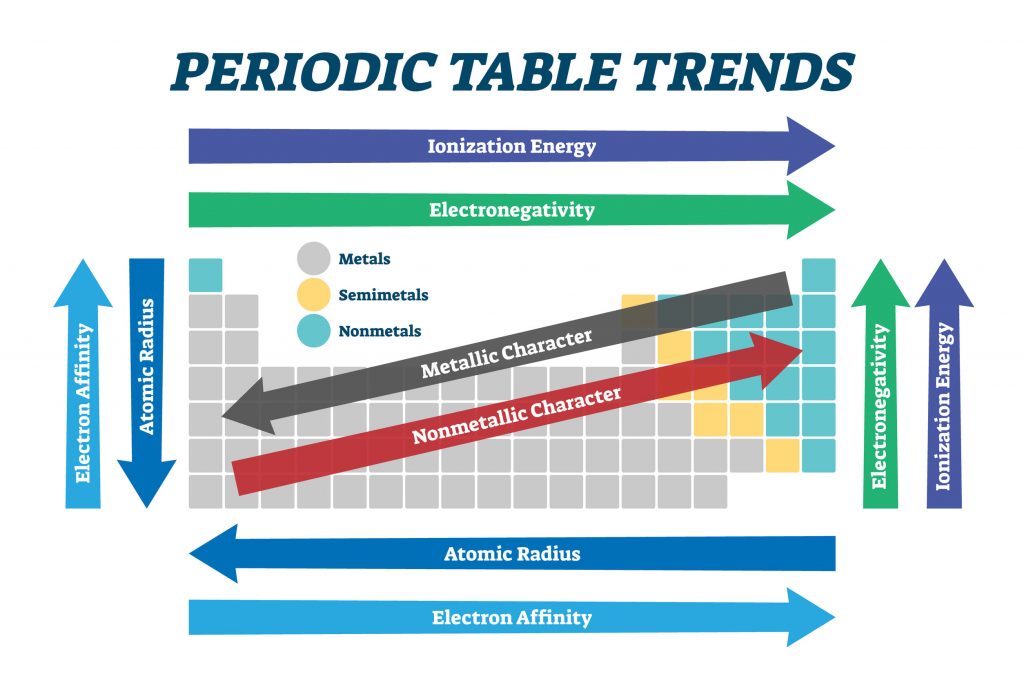
Chemists have always looked for ways of arranging the elements to reflect the similarities between their properties. The modern table lists the elements in order of increasing atomic number (the number of protons in the nucleus of an atom). Historically, however, relative atomic masses were used by scientists trying to organise the elements. This was mainly because the idea of atoms being made up of smaller sub-atomic particles (protons, neutrons and electrons) had not been developed. The basis was well established and even used to predict the properties of undiscovered elements long before the concept of the atomic number was developed.
Explore this amazing interactive version to discover what the elements are used for.

