pH is the abbreviation of the potential of Hydrogen and is the measure of the concentration of hydrogen ions in a water based substance. pH is arranged in a scale with acidic solutions at the low end and alkaline solution at the high end. Water is neutral and is in the middle of the scale.
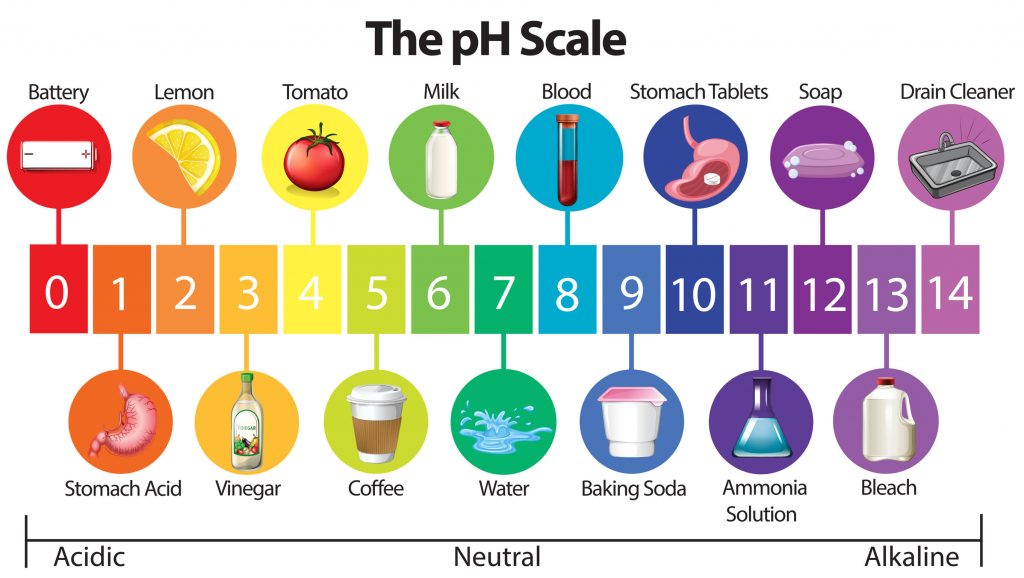
This diagram shows the pH ranges from 0 to 14, with 7 being neutral. pH of less than 7 indicate an acid and a pH of greater than 7 indicates a base.
pH is an important indicator of water that is changing chemically. pH is reported in “logarithmic units”. Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six.
Watch this video to find out more
pH activities
Have some fun in the kitchen and make your own pH indicator solution. Red cabbage juice make a natural pH indicator that changes colors according to the acidity of the solution. Red cabbage juice indicators are easy to make and can be used to make your own pH paper strips too.

